UIC researchers identify new process to produce ammonia with a much smaller carbon footprint
Text block
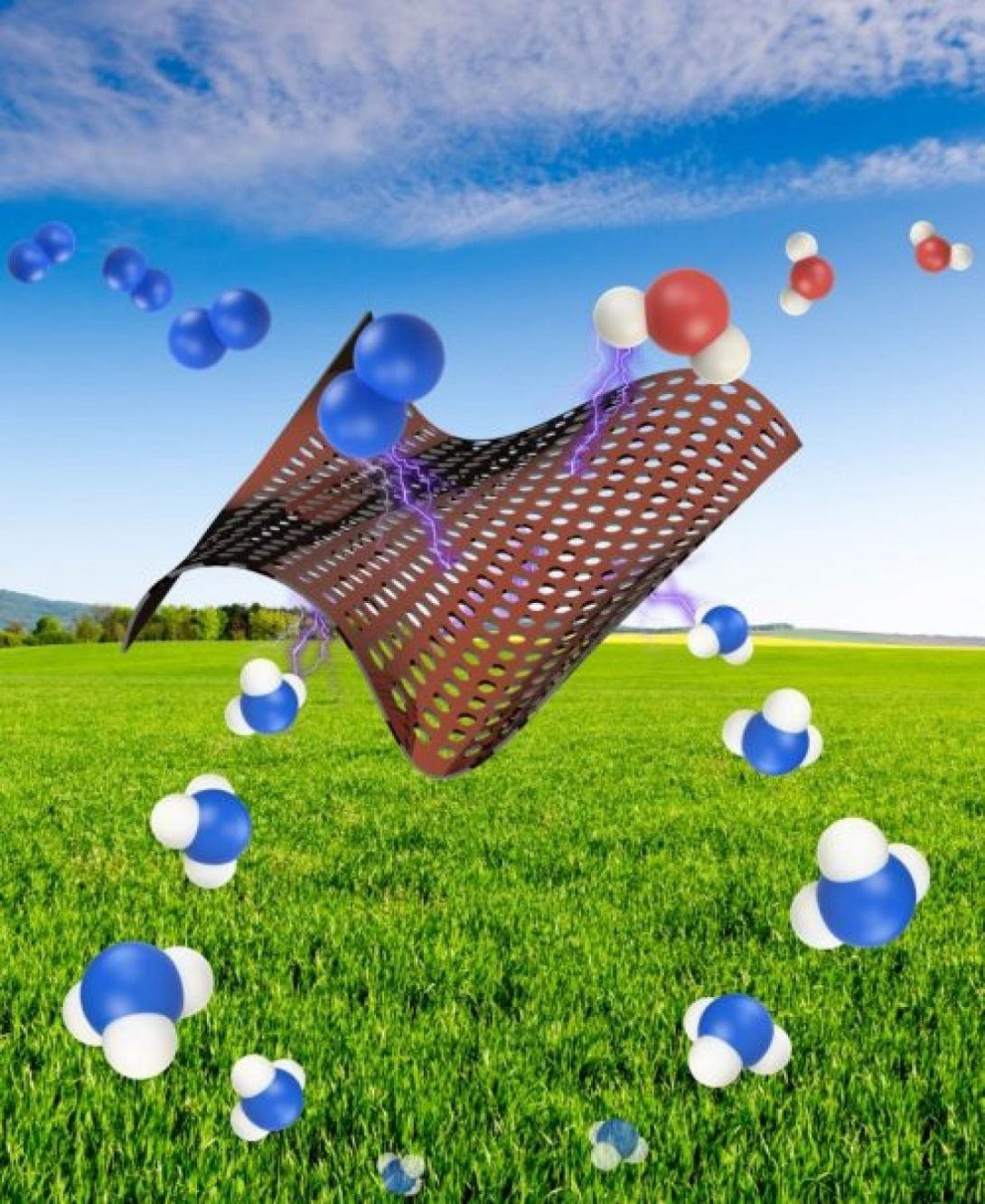
Ammonia is the second most commonly produced chemical in the world and an important component of most fertilizers, but current industrial processes to make ammonia produce several millions of tons of carbon dioxide–a potent greenhouse gas–each year.
Now, researchers led by Meenesh Singh, assistant professor of chemical engineering at the University of Illinois Chicago College of Engineering, describe a new process to produce ammonia with a potentially much lower carbon footprint. They report their findings in the journal ACS Catalysis.
Nitrogen gas is one of the components used to make ammonia, but because nitrogen bonds in nitrogen gas are very stable, a lot of energy is needed to break them so the nitrogen can bind to hydrogen to produce ammonia.
“Current methods to make ammonia from nitrogen are very energy-intensive and require the burning of fossil fuels to generate enormous amounts of heat, and this produces a lot of greenhouse gas as a byproduct,” said Singh.
Singh and colleagues have developed a new method to produce ammonia that relies on the use of a mesh screen coated in copper – a catalyst that helps bind nitrogen to hydrogen to make ammonia. The electrification of the screen helps drive the reactions.
Pure nitrogen gas is pushed through the screen and then interacts with water, which provides the hydrogen. Even though Singh’s process uses similar amounts of energy compared to the traditional process, it requires far less fossil fuels than traditional methods – just enough to electrify the screen. “The electricity can come from solar or wind energy, which would really make a huge difference in reducing greenhouse gas emissions,” said Singh. “Even modern electricity-generating powerplants are highly efficient, and if the grid is powered conventionally, our process still uses less fossil fuels and generates less harmful greenhouse gases than conventional ammonia production.”
Currently, Singh’s process produces 20% ammonia and 80 percent hydrogen gas. “We are hoping to increase the production of ammonia, but our early efforts so far are promising, and the savings in the carbon emissions are still significant if you were to scale up our process to produce large amounts of ammonia,” Singh said.
A provisional patent for the new process has been filed by the UIC Office of Technology Management.
Singh’s group is now looking at using air – instead of purified nitrogen gas – as a source of nitrogen for producing ammonia using their unique method. “Using air would give us even more savings when it comes to greenhouse gases because we’re using readily available air instead of nitrogen gas, which needs to be purified and bottled.”
Nishithan C. Kani and Aditya Prajapati of the University of Illinois at Chicago and Brianna Collins and Jason Goodpaster of the University of Minnesota are co-authors on the paper.