Research team looks to up the voltage in battery tech
Text block one
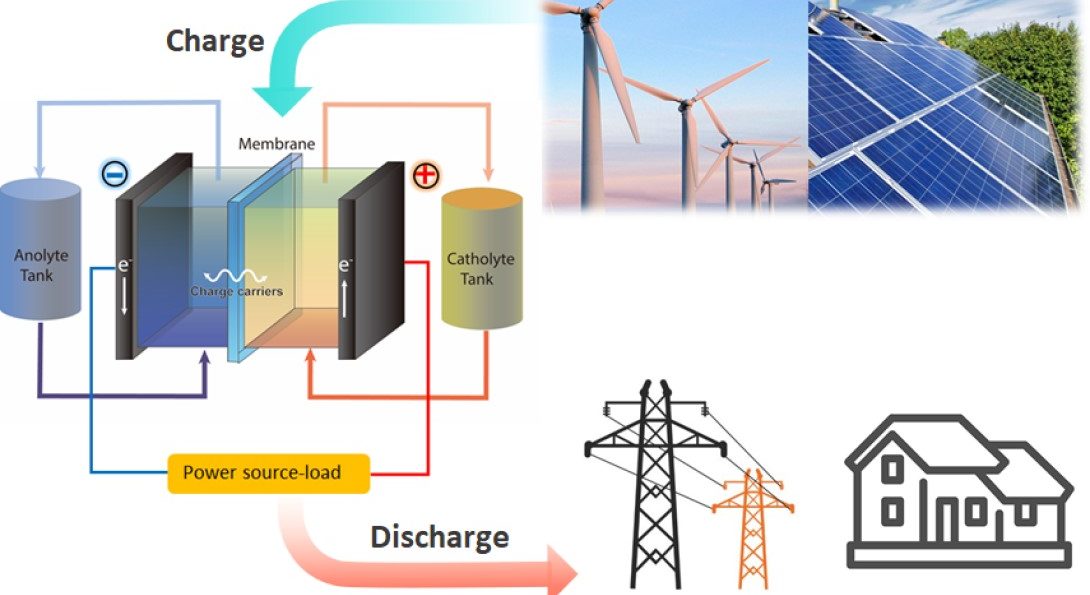
When most people picture batteries, they imagine the small tubes used to power remote controls and smoke detectors. But in recent years, scientists have started to think up bigger uses for new liquid flow batteries. Much bigger.
Liquid flow batteries, officially known as redox flow batteries, or RFBs, are rechargeable batteries that store electrical energy in two electrolyte tanks that can be circulated using pumps. Traditional lithium-ion batteries keep this material in confined spaces inside the battery and have safety issues when scaled up. In contrast, scientists believe they can build RFBs large enough to be used in power plants fueled by wind or solar energy.
One of the major hurdles that still need to be tackled before renewable energy can become more prolific and useful across the world is large grid-scale batteries have to be developed that can efficiently capture and store all this energy and distribute it across a power grid.
Before large operations like these can become a reality, researchers need to improve the performance and bring down the costs of many of the key components in RFBs. This is where Sangil Kim, UIC assistant professor of chemical engineering, and his team in the Molecular Transports in Nanopores Lab hope to contribute.
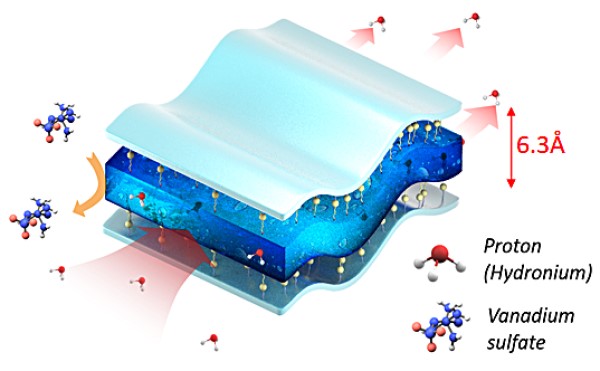
Kim said that vanadium redox flow batteries have garnered a lot of attention in the green energy community as a potential large-scale energy storage system for the renewable energy industry because of their high efficiency, absence of stress build-up in electrodes, easy heat management, and safe operation. As a key component in redox flow batteries, ion exchange membranes should have low vanadium permeability for the separation of the vanadium species in the catholyte and anolyte solution while allowing selective transport of charge-carrier ions, which is known as the tradeoff between ion selectivity and conductivity. But commercial ion exchange membranes used in vanadium redox flow batteries are too expensive and need to overcome the tradeoff to achieve high energy efficiencies of energy storage system.
Kim and his colleagues designed and optimized several nanoporous biphenyl membranes for use in vanadium redox flow batteries. They are based on sulfonated aromatic polymers that can overcome the tradeoffs between ion selectivity and conductivity. Their testing showed that the new membranes outperformed all other ion exchange membranes developed for these systems to date, Kim said, which is a major step toward making redox flow batteries a feasible part of a renewable power system.
“We are in the middle of an energy transition to renewable energy from fossil fuels such as coal, oil, and natural gas,” Kim said. “I believe the development of a low cost, reliable energy-storage technology with high performance is of great interest to our society because it will allow us to better utilize the capability of intermittent renewable energies from solar and wind power.”
Moving forward, the researchers are continuing to work on optimizing the membrane material so they can get the most power for the least amount of cost. Kim added they are testing several different structures, including a thin nanocomposite membrane and an organic/inorganic hybrid model, both of which use this ion exchange membrane as a base material.
“Development of a low cost, reliable energy storage technology for large grid-scale applications that can enhance the efficiency and quality of electrical grid is critical right now,” PhD student Tongshuai Wang said.
Kim and his colleagues published their new work in Material Advances in a paper titled “Suppressing Vanadium Crossover Using Sulfonated Aromatic Ion Exchange Membranes for High Performance Flow Batteries.” Funding for the project was provided by the the Interfacial Engineering Program of National Science Foundation and UIC Provost’s Graduate Research Award Program.
Additional authors include Dr. JunYoung Han, Dr. Kihyun Kim, Andreas Munchinger, Yuchen Gao, Dr. Alain Farchi, Dr. Yoong-Kee Choe, Professor Klaus-Dieter Kreuer, and Professor Chulsung Bae.