Paper looks to unlock water-energy membrane nexus
Text block one
Humans have been using water as a source of power for centuries from watermills grinding flour in mills to rivers flowing through massive hydroelectric damns to power whole cities.
Engineers in the 21st century are hoping to unlock a new way to harvest energy from water by using high-tech membranes. These same membranes could also be used to remove chemicals or other impurities from the same water.
Chemical engineering Associate Professor Sangil Kim said water and energy are two of the most pressing problems facing the world and membrane technology is a promising process at the water-energy nexus.
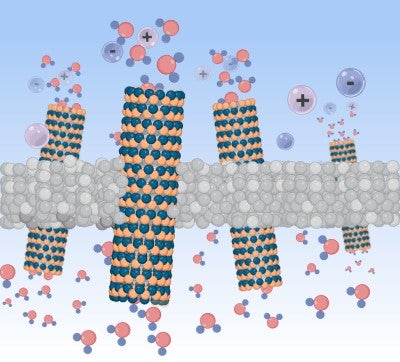
Because of this promise, Kim and his team wanted to investigate ion and water transports in charged 1D nanotubes to establish a fundamental understanding of ionic transport at a molecular scale. He added this transport in nanopores is a crucial physicochemical process for bringing these membranes from the lab out into the real world.
“Designing, evaluating, and developing next-generation membrane materials for desalination or energy conversion/storage systems requires a detailed understanding of ion transport in charged nanopores,” Kim said.
In pursuit of this understanding, Kim and his team decided to shed some light on this topic with a research paper in the journal Materials Today titled “Intrinsic ion transport of highly charged sub-3-nm boron nitride nanotubes.”
Kim noted that 1D nanotubes such as carbon nanotubes have been widely used to fabricate nanopore devices to investigate molecular transport in the past, but there is an ongoing debate about the transport mechanism of water and ions in highly charged nanotubes because of a lack of available data.
For the paper, the team performed an accelerated evaluation of ion transport properties through charged 1D nanotubes, looked to establish a comprehensive understanding of ion transport mechanisms in charged nanopores, and created new, highly ion-selective nanoporous materials for potential applications in membrane-based ion separation.
“We anticipate that the improved understanding of ion-transport mechanisms in highly surface-charged systems will lead to advancements in water purification, desalination, renewable energy production, energy storages, electro-osmotic micropumps, and DNA sequencing applications,” Kim said.
The research has been supported by the Advanced Manufacturing program of the National Science Foundation. In addition to Kim, graduate students Aaditya Pendse, Kun Wang, Donglin Li, and Tongshuai Wang were coauthors of the article. Other non-UIC co-authors included Semih Cetindag, Richard Castellano, Da-Chi Yang, and Professor Jerry Shan at Rutgers University.